Authors
Brian F. Quach BS1, Alexander Hayden MS, PA-C1,2, Eric Nohelty BS, CHSOS3,4, Andrew J. Eyre MD, MS3,4,5
1Frank H. Netter MD School of Medicine at Quinnipiac University, North Haven, CT
2William W. Backus Hospital, Department of Surgery, Norwich, CT
3STRATUS Center for Medical Simulation, Boston, MA
4Brigham and Women’s Hospital, Department of Emergency Medicine, Boston, MA
5Harvard Medical School, Boston, MA
Conflict of Interest Statement
The authors of this manuscript declare no conflicts of interest. Innovations were designed at the STRATUS Center for Medical Simulation when author BFQ was employed there.
Corresponding Author
Brian F. Quach, BS, Frank H. Netter MD School of Medicine at Quinnipiac University, North Haven, CT
(Email: Brian.Quach23@gmail.com)
Brief Description
Traumatic liver injuries are among the most common life-threatening emergencies observed in patients worldwide, most often resulting from blunt force trauma or penetrating injuries (Coccolini et al., 2020; H. Jiang & Wang, 2012). Due to the liver’s essential functions, dense vascular supply, and large surface area, poor management of these injuries can lead to severe complications and high mortality (Arık et al., 2013; Gao et al., 2003; Ozougwu, 2017; Taghavi & Askari, 2023). Given that quick and appropriate action is required to maximize favorable patient outcomes, surgical practitioners must receive efficient training to ensure they can repair these injuries competently. Medical simulation offers practitioners a psychologically safe educational space to improve their psychomotor skills. Historically, animal models have been a gold standard for surgical training in medical simulation (Cordero et al., 2011; DeMasi et al., 2016; Loh et al., 2017). However, animal products (AP) may not always be readily available and may carry ethical and practical considerations (Kadima et al., 2006; Parra-Blanco et al., 2013). In addition to ethical issues, the need to repurchase APs repeatedly can be financially burdensome. These concerns underscore the need for alternative training methods in the development of surgical skills. Using readily available materials, we designed a reusable and cost-effective model that provides realistic feel and tissue response to train surgical practitioners.
Introduction
Uncontrolled hemorrhages are one of the major causes of death amongst trauma patients. Given the liver’s size, vascular supply, and relatively fragile parenchyma, it is a common source of life-threatening hemorrhage (Coccolini et al., 2020; Gao et al., 2003). Bleeding from liver injury is associated with a high mortality rate and can result from various mechanisms, with the most acute and life-threatening cases typically involving penetrating trauma or blunt force injury (Jin et al., 2012; Keizer et al., 2020; Slotta et al., 2013; Tarchouli et al., 2018). Given the time-sensitive nature of such cases, it is crucial for surgical practitioners to be thoroughly trained in the prompt management of these injuries. With medical simulation, evidence-based teaching methods can be used to improve practical skills and knowledge in managing complex injuries like liver trauma (Delingette & Ayache, 2005; Rashidian et al., 2020).
While APs are commonly used in surgical procedure training, there are associated ethical, financial, and logistical challenges (Broom, 2010; Kadima et al., 2006; Parra-Blanco et al., 2013). Firstly, specialty APs bought in bulk can be expensive due to the careful and time-demanding harvesting process. In some cases, APs can carry diseases and environmental hazards due to unhygienic shipping and handling practices. Second, as APs spoil quickly after use, multiple teaching sessions will require the purchase of new APs for each cohort, contributing to overspending. Lastly, with growing emphasis on animal welfare advocacy and cultural considerations, the use of APs may conflict with individual morals, especially if the use of APs in training is not an absolute necessity.
In recent years, technological innovations like extended reality have been introduced to shift the paradigm of medical education and surgical training (Preibisch et al., 2024; Suresh et al., 2022; Toni et al., 2024; Woodall et al., 2024). However, these new methods have limitations regarding how learners can fully refine and develop their psychomotor skills (Co et al., 2023; Woodall et al., 2024). Despite being relatively costly, three-dimensional printing and silicone modeling in medical simulation has enabled learners to hone surgical skills using anatomically precise models (Jiang et al., 2024; Tenewitz et al., 2021; Nagamoto et al., 2023). We propose an innovative approach that utilizes the traditional method of hands-on surgical training, enabling learners to fully cultivate these skills with a liver-shaped simulator. In this manuscript, we describe the creation of a low-cost, easily made simulator to replicate traumatic liver injuries.
Objective
This project sought to create a cost-effective, alternative to animal products to train surgeons in repairing liver injuries with techniques such as primary suture repair, ligation and intrahepatic tamponade. As an AP alternative, we aimed to create a model reusable across multiple simulation sessions. A key goal of this project is to highlight the importance of simulation training for liver injury management, given the high number of traumatic liver injury cases in both the United States and globally (Chien et al., 2013; Taghavi & Askari, 2023).
Model Design Methods
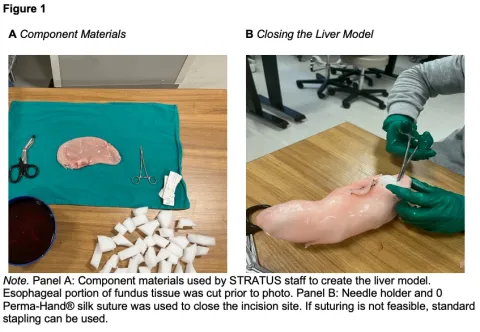
The cost and materials to create the simulated liver model are presented below (Table 1). Full instructions for this model can be found in Appendix A. Upholstery foam was cut into cubes, and red food coloring was mixed with warm water. These products provided the liver model with structural integrity and the color of living tissue, respectively. The fundus tissue (Replaceable Fundus Tissue 10-Pack, 2024) was chosen as the model’s foundation due to its likeness in shape to the liver. The esophageal attachment of the fundus tissue was repurposed to simulate the falciform ligament by attaching it with twine to divide the model into the right and left lobes. Other required instruments included trauma shears, a needle holder, and sutures.
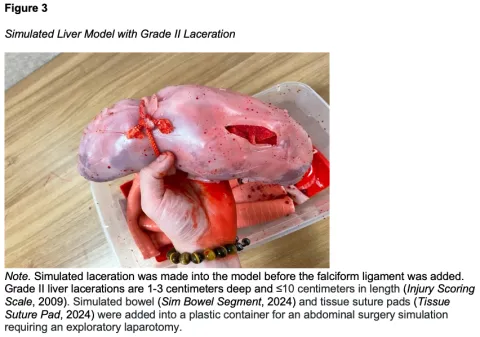
After the foam cubes and red solution were prepared, we began the model making process. The esophageal attachment of the fundus was removed using trauma shears, and foam cubes werde inserted into the fundus tissue (Figure 1A). As cubes were inserted, the tissue adopted the shape of a liver, with the area distal to the opening appearing smaller than the more proximal region. This allowed a clear distinction between the right and left lobes to place the twine and the esophageal attachment, as they both differ in size anatomically. A realistically size falciform ligament was simulated by utilizing twine to bind the esophageal attachment. After filling, the opening was sutured shut using a needle holder and 0 Perma-Hand® silk suture (PERMA-HANDTM Silk Suture, 2022) (Figure 1B). Once closed, twine was tied on one end of the model creating the border to separate the right and left lobes of the liver made by the uneven distribution of foam for durability. The liver was then left to soak in the red solution for 10-15 minutes to give the appearance of living tissue. Finally, the esophageal attachment was bound tightly above the twine to simulate the falciform. This step is performed after soaking, as the falciform ligament is a different color biologically compared to the remainder of the liver. This completed construction and overall construction for this liver model should take approximately 30 minutes, including the time for soaking (Figure 2). The liver model was then simulated to have a grade II laceration for repair simulation (Figure 3).

Results
We created a sustainable and reusable liver model for surgical skill training using materials easily obtained in our simulation lab. The cost to make one liver model is $116.11 USD. The model allows for the learner to practice technical skills during surgery simulation that include, but are not limited to, laceration repair and perihepatic packing with. For the initial liver model, we created a grade II laceration for suture repair using an #11 blade scalpel. However, there is potential for customization, with future iterations including mechanisms of injury such as impalement or gunshot with retained bullet. For high fidelity surgery simulations, this liver can be placed inside of a full-body mannequin and paired with other simulated organs to create a more immersive surgical experience for learners.
Discussion
The net cost for the construction of one liver model was $116.11 USD, with the most expensive component being the fundus tissue. For this reason, the fundus tissue may not be readily accessible, and cheaper alternatives can be made using silicone as the mold of a human liver. Foam can also be used to fill the interior of the silicone model, although some silicone may become trapped between the foam layers in this design. If necessary, for repair, additional silicone may be applied to the open lesions for model closure.
With this simulator, surgical practitioners can practice the fine motor skills essential for open surgery injury repair, like suturing. Severity of the injury grade can be manipulated accordingly (Injury Scoring Scale, 2009). Additional customization of this model can include the addition of liver tumors by adding small Styrofoam spheres to simulate semicircular liver projections and a silicone skin layer. For this model, Styrofoam is preferred as it can maintain its shape after infiltration by a biopsy needle or ablation antenna. With these additions, learners can practice minimally invasive surgical procedures such as tissue biopsy, hepatectomy, and microwave tumor ablation. The liver’s major blood vessels, the hepatic portal vein and hepatic artery, can be simulated with rubber tubing spanning from the superior to the inferior aspects of the model. With the addition of these structures, learners can practice more advanced surgical procedures like portal vein and hepatic artery ligations.
This liver model can be integrated into a container with simulated abdominal organs for use with the Fundamentals of Laparoscopic Surgery (FLS) trainer for a minimally invasive approach to surgical repair. Given that laparoscopic surgery is a commonly required skill in addition to traditional open surgery techniques (Carr et al., 2018; Patil et al., 2024), combining this model with the FLS trainer enables the learner to practice core techniques associated with laparoscopic surgical repair. As this is an alternative to AP, there is a decreased risk of disease transmission and environmental hazards when pairing this model with a multi-purpose FLS trainer. This model shows great promise for high-acuity surgical simulations. By adding a red fluid mixture inside the simulated liver, it can be lacerated to create a team-based scenario focused on managing actively hemorrhaging liver injuries.
Limitations of Simulator
There are some limitations with this simulator to consider. First, it does not fully replicate the complexity of responding to a trauma with patients who have sustained high-grade liver injuries requiring emergent operative intervention. In a case-based scenario, a simulation lab can potentially work with a script, actors, and mock clinical and operating rooms, but the true pressure of a real trauma situation cannot be fully replicated with our model.
Second, this initial liver model is designed to simulate a grade II laceration in an otherwise healthy liver and does not replicate other liver conditions which might result in a more complicated repair, such as cirrhosis, hepatic steatosis, hepatitis, or carcinomas. Additionally, the size of this simulated liver does not accurately replicate the liver of a larger adult, child, or infant, limiting the simulator’s use for trauma surgeons in training. This limitation could, however, be addressed by adjusting the size of the fundus tissue and foam padding. For pediatric livers, models could utilize a smaller tissue sample and less foam padding. For larger adult livers, multiple layers of fundus tissue and foam padding may be needed to expand the surface area of the existing model, though this could increase the cost of model construction.
The lack of an active circulatory system with pulsatile arterial spray and venous obstacles limits realism. In a trauma situation, the operator would need to take careful steps around these vital structures to avoid introducing more damage. This is particularly relevant vascular injuries due to a grade III-IV injury (Injury Scoring Scale, 2009). In these situations, the surgeon must manage both parenchymal tears and vascular injuries, which can complicate repair due to blood loss.
Similarly, our model is limited by the lack of simulated bile ducts. Significant structures like the common hepatic and bile duct are commonly used landmarks for gallstone removal. Without the addition of rubber tubing to simulate these structures, the ability to practice these skills on the simulated liver model is significantly limited. Lastly, this model does not include the gallbladder, which is attached inferiorly to the liver. We solely focused our efforts into constructing the liver. To address this, a gallbladder and associated ducts can be simulated with a balloon and tubing on the inferior aspect of the liver.
Finally, this model has not been evaluated by subject matter experts. The feedback from subject matter experts is vital to improve the model’s utility and efficacy in surgical laceration repair training. At the time of this writing, we have not obtained expert feedback due to logistical challenges. Future simulation studies and training sessions utilizing this model should seek to gather expert feedback on this model’s resemblance to a real liver and comparison to APs as an educational tool.
Conclusion
In summary, a simulated liver was created using materials available in our medical simulation center. The model is a viable and reusable alternative to animal liver, addressing ethical and practical considerations associated with the use of these items. The customizability of the model allows for the practice of managing different mechanisms of liver injury.
References
Arık, M. K., Tas, S., Özkul, F., Şahin, H., Karatağ, O., & Karaayvaz, M. (2013). Laparoscopic Repair of Combined Right Diaphragm and Liver Injuries with a Sharp Object: A Case Report. Case Reports in Surgery, 2013, 1–3. https://doi.org/10.1155/2013/209494
Broom, D. M. (2010). Animal welfare: an aspect of care, sustainability, and food quality required by the public. Journal of Veterinary Medical Education, 37(1), 83–88. https://doi.org/10.3138/jvme.37.1.83
Carr, B. M., Lyon, J. A., Romeiser, J., Talamini, M., & Shroyer, A. L. W. (2018). Laparoscopic versus open surgery: a systematic review evaluating Cochrane systematic reviews. Surgical Endoscopy, 33(6), 1693–1709. https://doi.org/10.1007/s00464-018-6532-2
Chien, L., Lo, S., & Yeh, S. (2013). Incidence of liver trauma and relative risk factors for mortality: A population-based study. Journal of the Chinese Medical Association, 76(10), 576–582. https://doi.org/10.1016/j.jcma.2013.06.004
Co, M., Chiu, S., & Cheung, H. H. B. (2023). Extended reality in surgical education: A systematic review. Surgery, 174(5), 1175–1183. https://doi.org/10.1016/j.surg.2023.07.015
Coccolini, F., Coimbra, R., Ordonez, C., Kluger, Y., Vega, F., Moore, E. E., Biffl, W., Peitzman, A., Horer, T., Abu-Zidan, F. M., Sartelli, M., Fraga, G. P., Cicuttin, E., Ansaloni, L., Parra, M. W., Millán, M., DeAngelis, N., Inaba, K., Velmahos, G., . . . Catena, F. (2020). Liver trauma: WSES 2020 guidelines. World Journal of Emergency Surgery, 15(1). https://doi.org/10.1186/s13017-020-00302-7
Cordero, A., Del Mar Medina, M., Alonso, A., & Labatut, T. (2011). Stapedectomy in sheep. Otology & Neurotology, 32(5), 742–747. https://doi.org/10.1097/mao.0b013e31821ddbc2
Delingette, H., & Ayache, N. (2005). Hepatic surgery simulation. Communications of the ACM, 48(2), 31–36. https://doi.org/10.1145/1042091.1042116
DeMasi, S. C., Katsuta, E., & Takabe, K. (2016). Live animals for preclinical medical student surgical training. Editorial Journal of Surgery, 5, PMCID: PMC5509225. https://pmc.ncbi.nlm.nih.gov/articles/PMC5509225/
Gao, J., Du, D., Zhao, X., Liu, G., Yang, J., Zhao, S., & Lin, X. (2003). Liver trauma: experience in 348 cases. World Journal of Surgery, 27(6), 703–708. https://doi.org/10.1007/s00268-003-6573-z
Injury scoring scale: A resource for trauma care professionals. (2009). The American Association for the Surgery of Trauma. Retrieved April 8, 2025, from https://www.aast.org/resources-detail/injury-scoring-scale
Jiang, H., & Wang, J. (2012). Emergency strategies and trends in the management of liver trauma. Frontiers of Medicine, 6(3), 225–233. https://doi.org/10.1007/s11684-012-0186-6
Jiang, Y., Jiang, H., Yang, Z., & Li, Y. (2024). The current application of 3D printing simulator in surgical training. Frontiers in Medicine, 11. https://doi.org/10.3389/fmed.2024.1443024
Jin, W., Deng, L., Lv, H., Zhang, Q., & Zhu, J. (2012). Mechanisms of blunt liver trauma patterns: An analysis of 53 cases. Experimental and Therapeutic Medicine, 5(2), 395–398. https://doi.org/10.3892/etm.2012.837
Kadima, K., Hassan, A., Remi-Adewumi, B., Awasum, C., & Abubakar, M. (2006). Animal models in surgical training: choice and ethics. Nigerian Journal of Surgical Research, 7(3). https://doi.org/10.4314/njsr.v7i3.12292
Keizer, A. A., Arkenbosch, J. H. C., Kong, V. Y., Hoencamp, R., Bruce, J. L., Smith, M. T. D., & Clarke, D. L. (2020). Blunt and Penetrating Liver Trauma have Similar Outcomes in the Modern Era. Scandinavian Journal of Surgery, 110(2), 208–213. https://doi.org/10.1177/1457496920921649
Loh, C. Y. Y., Wang, A. Y. L., Tiong, V. T. Y., Athanassopoulos, T., Loh, M., Lim, P., & Kao, H. (2017). Animal models in plastic and reconstructive surgery simulation—a review. Journal of Surgical Research, 221, 232–245. https://doi.org/10.1016/j.jss.2017.08.052
Nagamoto, T., Kubono, H., Kawamura, M., & Suzuki, K. (2023). A custom-made vitreoretinal surgical simulator using a silicone mold. BMC Ophthalmology, 23(1). https://doi.org/10.1186/s12886-023-03070-5
Ozougwu, J. C. (2017). Physiology of the liver. International Journal of Research in Pharmacy and Biosciences, 4(8), 13–24.
Parra-Blanco, A., González, N., González, R., Ortiz-Fernández-Sordo, J., & Ordieres, C. (2013). Animal models for endoscopic training: do we really need them? Endoscopy, 45(06), 478–484. https://doi.org/10.1055/s-0033-1344153
Patil, M., Gharde, P., Reddy, K., & Nayak, K. (2024). Comparative analysis of laparoscopic versus open procedures in specific general surgical interventions. Cureus. https://doi.org/10.7759/cureus.54433
PERMA-HANDTM Silk Suture. (2022). J&J Med Tech. Retrieved April 8, 2025, from https://www.jnjmedtech.com/en-US/product/perma-hand-silk-suture
Rashidian, N., Vierstraete, M., Alseidi, A., Troisi, R. I., & Willaert, W. (2020). Surgical education interventions in liver surgery: a systematic review. Updates in Surgery, 72(3), 583–594. https://doi.org/10.1007/s13304-020-00766-x
Replaceable Fundus Tissue 10-Pack. (2024). Simulab. Retrieved April 8, 2025, from https://simulab.com/products/replaceable-fundus-10-pack
Sim Bowel Segment. (2024). Simulab. Retrieved April 8, 2025, from https://sim-vivo.com/simbowel-segment.html
Slotta, J. E., Justinger, C., Kollmar, O., Kollmar, C., Schäfer, T., & Schilling, M. K. (2013). Liver injury following blunt abdominal trauma: a new mechanism-driven classification. Surgery Today, 44(2), 241–246. https://doi.org/10.1007/s00595-013-0515-7
Suresh, D., Aydin, A., James, S., Ahmed, K., & Dasgupta, P. (2022). The Role of Augmented Reality in Surgical Training: A Systematic review. Surgical Innovation, 30(3), 366–382. https://doi.org/10.1177/15533506221140506
Taghavi, S., & Askari, R. (2023, July 17). Liver Trauma. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK513236/
Tarchouli, M., Elabsi, M., Njoumi, N., Essarghini, M., Echarrab, M., & Chkoff, M. R. (2018). Liver trauma: What current management? Hepatobiliary & Pancreatic Diseases International, 17(1), 39–44. https://doi.org/10.1016/j.hbpd.2018.01.013
Tenewitz, C., Le, R. T., Hernandez, M., Baig, S., & Meyer, T. E. (2021). Systematic review of three-dimensional printing for simulation training of interventional radiology trainees. 3D Printing in Medicine, 7(1). https://doi.org/10.1186/s41205-021-00102-y
Tissue suture pad. (2024). Simulab. Retrieved April 8, 2025, from https://simulab.com/products/tissue-suture-pad?_pos=2&_sid=41bf2c55c&_ss=r
Toni, E., Toni, E., Fereidooni, M., & Ayatollahi, H. (2024). Acceptance and use of extended reality in surgical training: an umbrella review. Systematic Reviews, 13(1). https://doi.org/10.1186/s13643-024-02723-w
Woodall, W. J., Chang, E. H., Toy, S., Lee, D. R., & Sherman, J. H. (2024). Does extended reality simulation improve Surgical/Procedural learning and patient outcomes when compared with standard training methods? Simulation in Healthcare the Journal of the Society for Simulation in Healthcare, 19(1S), S98–S111. https://doi.org/10.1097/sih.0000000000000767
Appendix A
Model Making Instructions
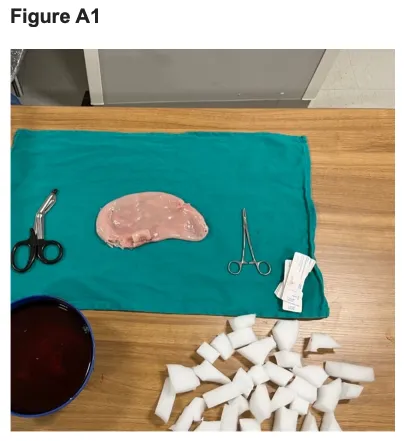
1. Obtain and cut upholstery foam into cubes utilizing trauma shears (Figure A1).
2. Add red food coloring to warm water and place foam cubes in to create structural integrity and realistic color for the liver model.
3. Once obtained take artificial fundus material and remove esophageal attachment utilizing trauma shears this will be used later for mimicking falciform ligament (Simulab Corporation, Seattle, Washington, United States of America).
4. Shape fundus tissue into liver-like structure.
5. Insert foam cubes from step 2 into the esophageal opening of the newly formed liver like structure you will notice as the shaped fundus fills it will adopt a more realistic three-dimensional liver like structure.
6. Once fundus is filled and approximates a liver like structure, use suture and needle driver to close the esophageal opening (Figure A2) (Ethicon Inc., Raritan, New Jersey, United States of America).

7. Take twine and cut to length to be able to tie it at a point roughly central on the liver model to simulate left and right lobes.
8. Soak liver model in solution of warm water to help dye it into a more realistic color, allow it to soak for 15 minutes minimum. Do not soak previously removed esophageal attachment.
9. After the model has been soaked, remove and allow to dry.
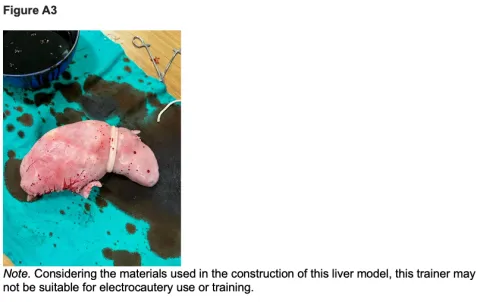
10. Place previously removed esophageal attachment atop twine utilized for separating left and right lobes. This will mimic the falciform ligament which is a different color in real human liver tissue (Figure A3).