Authors
Karen Murley1, Sripriya Srinivas1, Ayonica Bhattacharya1, James Garner1, Hunter Wagnon1, Cheryl Gomillion, PhD1, Michael Croyle2, Aimee T. Martin, MD2
1University of Georgia College of Engineering, Athens, GA
2Augusta University / University of Georgia Medical Partnership, Athens, GA
Conflict of Interest Statement
The authors of this manuscript declare no conflicts of interest.
Corresponding Author
Aimee T. Martin, Augusta University / University of Georgia Medical Partnership, Athens, GA
(Email: aimeem@uga.edu)
Brief Description
Health professions education programs are increasingly incorporating manikin-based simulation (MBS) into their curriculum, and access to effective moulage is critical to providing realistic high-fidelity scenarios (Huang et al., 2012). Simulation effectiveness rests partly on maintaining a fiction contract with the learner (Issenberg et al., 2005). Poorly constructed moulage can break down the fiction contract, detract from the learning experience, and inadvertently demonstrate incorrect physical exam findings to clinically inexperienced learners (INACSL Standards Committee, 2016; Rudolph et al., 2014). Our curriculum contains several MBS cases that feature the exam finding of lower extremity edema. We experienced significant difficulty finding realistic lower extremity edema moulage applications. We collaborated with our institution’s undergraduate engineering capstone program to design and manufacture a low-cost, highly realistic moulage application mimicking lower extremity pitting edema. Student and faculty survey findings were overwhelmingly positive. Survey participants highlighted the improved realism of the rebound and pitting effect, more accurate tactile response, and greater usability compared to the commercially available foam-based device previously used.
Introduction
Lower extremity edema is a critical physical exam finding associated with common conditions such as deep vein thrombosis, heart failure, and kidney disease. The ability to recognize and grade edema is an essential skill for healthcare providers, as it assists in timely diagnosis and the selection of appropriate treatments. As health professions students are increasingly exposed to patient care experiences through standardized patients (SP) and manikin-based simulation (MBS) cases during preclinical training, it is imperative that these experiences mimic real-life patient presentations as closely as possible to ensure adequate preparation for actual patient encounters. Poorly constructed moulage can lead to a breakdown of the fiction contract, confusion among experienced learners who expect specific physical exam findings, and demonstration of incorrect findings to inexperienced learners.
An online search for wearable edema moulage accessories revealed models with significant limitations in realism. These limitations include short length (ankle edema only), poor facsimiles of skin appearance and texture, and use of memory foam, creating an unusual tubular appearance to the extremity. The tactile experience of the models we tested did not simulate that of human skin, and the pitting of the material rebounded too quickly in a non-physiologic manner. Additionally, available skin tone options were limited in wearable commercially available products. Driven by these limitations, we endeavored to develop a device that looks, feels, and acts like edematous human tissue to provide realistic exam findings for both inexperienced and advanced clinical learners.
Our objective was to create a model that was durable, easily reproducible without a need for specialized equipment, and cost-effective. The resulting device is a layered application utilizing tattoo skin, silicone, nanotape, and maltose gel. The tattoo skin serves as the visible outer layer, selected to match the desired skin tone. The nanotape forms a sealed, leak-proof pocket that contains the maltose gel. A thin silicone sheet backing prevents adhesion to the leg when applied. All layers are stitched together, and elastic bandages are affixed to the sides. These are secured around the leg with hook-and-loop fasteners, allowing the device to be firmly attached to the limb with even pressure and no distortion. Our device fills a critical gap in medical education by realistically simulating pitting edema with a range of skin pigmentations.
Materials and Cost
This device can be created without need for specialized equipment and utilizes easily obtainable materials sourced online. The final material cost is approximately 83 USD per device (Table 1). The estimated time for one person to create one device is 4 hours, assuming all materials are readily available (Table 1).
Device design and construction
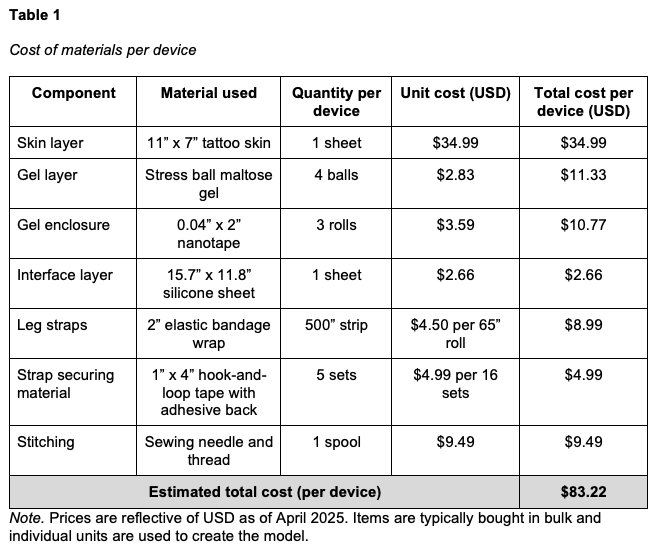
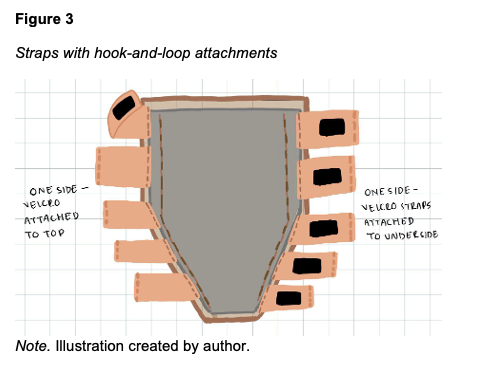
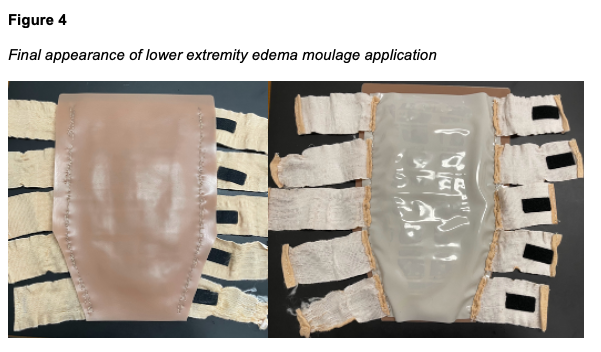
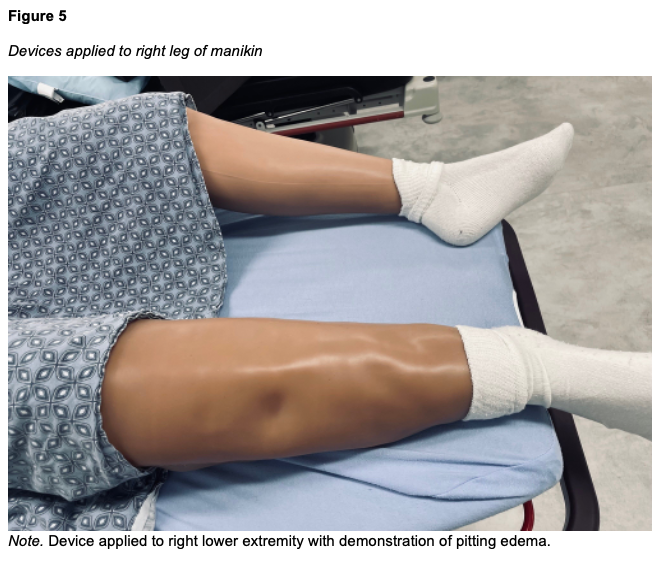
Five devices were created, three to fit Laerdal SimMan 3G/3G+ (Laerdal SimMan 3G, Laerdal Medical, Wappingers Falls, NY) and two to fit Gaumard Victoria (S2200 birthing simulator, Gaumard Scientific, Miami, FL). The outer “skin” layer is achieved using a 3mm thickness tattoo skin sheet in the desired skin tone. The 11” x 17” sheet is trimmed on one end to create a tapering effect that matches the narrowing contour of a human leg from the calf to the ankle. The second layer is an enclosure made from double-sided nanotape. This enclosure contains the maltose gel which forms the innermost layer of the device (Figure 1). The amount of maltose hydrogel used during production determines the simulated level of edema. The current model is designed to simulate Grade 2+ to 3+ edema, with an objective indentation depth of between 3 to 6 mm and a rebound time of between 15 and 30 s. A thin silicone sheet is used as the bottom layer, interfacing with the leg (Figure 2). Ten securing straps, five on each side, are sewn in place along the left and right edges of the device (Figure 3). These straps consist of 2” wide elastic bandage wrap cut-to-size, with opposing sections of hook-and-loop fasteners affixed to them. The fasteners allow the device to be securely attached around the manikin's leg with even pressure, avoiding bulging or raised edges. See Figures 4 and 5 for the finished appearance. See Supplemental Material for detailed instructions and material purchasing information.


Data Collection and Analysis
Objective testing
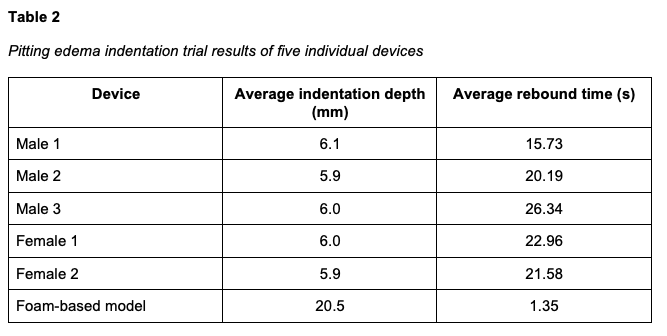
To establish objective performance metrics, we first determined pitting edema specifications based on established clinical guidelines (Calzon et al., 2023). We defined the target simulation parameters as follows: Grade 2+ edema was characterized by a 3–4 mm indentation with an approximate rebound time of 5–15 seconds. Grade 3+ edema was characterized by a 5–6 mm indentation with an approximate rebound time of 15–60 seconds.
We conducted 10 indentation trials across different areas of the lower leg for each device. Each indentation was pressed fully, and the resulting depth was recorded. Upon release, the rebound time was measured from the moment of release to full surface recovery. We also tested the commercially available foam-based leggings currently in use at our facility. All five of our manufactured devices performed within the target metrics, successfully mimicking Grade 3+ pitting edema, whereas the foam-based device displayed non-physiologic, near instantaneous rebound and demonstrated excessive depth of indentation. The results are summarized in Table 2.
Durability testing
Durability was assessed through two standardized protocols: palpation testing and attachment/removal testing. For palpation testing, we performed 100 repeated palpation cycles on each device to simulate frequent use during clinical training. All devices maintained structural integrity and continued to function as intended throughout the testing. For attachment/removal testing, each device was attached and removed from a manikin leg 30 times. We monitored the fit, material wear, and fastening strength throughout the process. While there was some minor loosening over time, the overall performance remained within acceptable limits given the expected frequency of use in the simulation center. The device maintained structural integrity and continued to function as intended over a period of six months when stored flat at room temperature (68°). They have not yet been tested beyond that time.
End-user survey
To assess realism and usability, we surveyed 15 participants, including 12 medical students, 2 physician faculty members, and 1 non-physician faculty member. The survey collected feedback on the visual realism, tactile feel, ease of use, and immersion provided by the new device compared to the current foam-based device. A 5-point Likert scale (1 = Not Effective, 2 = Somewhat Effective, 3 = Effective, 4 = Very Effective, 5 = Highly Effective) was used to assess feedback on both devices (Table 3).
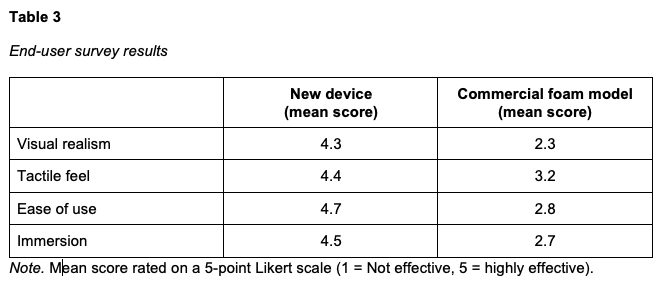
Feedback was overwhelmingly positive. Participants highlighted the improved realism of the rebound and pitting effect, a more accurate tactile response, and greater usability compared to the commercial foam model. Many noted that the device better represented actual pitting edema and offered a more immersive training experience. The simplicity of the attachment and improved durability were also favorably mentioned, further validating the design in meeting user needs. Representative comments included:
“Rebound time, pitting, and texture feels really good and much better than the foam one.”
“Bulkiness is pretty accurate and the increase in realism is really good.”
“Original just compresses, but the new device has an actual pit and rebounds too.”
“I like how it leaves a pit and rebounds pretty similar to actual pitting edema.”
“It is much easier to use than the current device.”
“This will be really useful for future simulations considering the improvements made.”
Discussion
This lower extremity edema moulage application is a simply applied manikin leg overlay designed to maximize fidelity. It provides realistic pitting edema, both visual and tactile, for healthcare learners in simulated patient care settings. This model has several advantages over currently commercially available edema applications. It covers the entire lower leg and tapers at the ankle, which facilitates the fitting a sock over the foot and ankle, thereby concealing the lower seam. Furthermore, it allows for edema measurements along the entire lower leg. The maltose core provides a realistic tactile sensation when pressure is applied and rebounds slowly, accurately mimicking the characteristics of real pitting edema. The design is easily modifiable to fit most simulators, is available in various skin tones, and can be adapted to increase or decrease the level of edema demonstrated. Additionally, it is durable, holding up well to repeated application and removal, which makes the initial time investment worthwhile. While we did not test this design on human actors, it has the potential to be applied to the leg of an SP to demonstrate pitting edema during SP encounters.
This model is highly cost effective, with an estimated material cost of 83 USD per device (166 USD per pair). Our research revealed that commercially available wearable extremity edema applications cost anywhere between 410 and 849 USD, with many of these models not covering the entire lower extremity. For comparison, the commercially available foam model used in our comparison testing cost 650 USD per pair.
Limitations of device
A primary limitation of this device is the time required for manufacturing (estimated to be 4 h). However, the durability testing demonstrated the device can be reused many times, mitigating the impact of the initial time investment over the product’s lifespan.
The fabric elastic straps are susceptible to stretching or tearing with multiple uses. Since they are not stitched into the nanotape layer, they can be replaced independently without compromising the main structure of the device. Alternatively, a continuous loop elastic system could be implemented instead of the hook-and-loop system, allowing users to pull the device over the foot onto the leg where it would be held in place by tension.
These devices need to be stored lying flat, as stacking them upright can cause the maltose gel to migrate to the dependent end, leading to an uneven appearance of edema. This can be rectified by laying the device flat and gently massaging the gel enclosure to maneuver the gel to a uniform thickness. Stacking the devices without a protective layer between them can lead to damage to the elastic straps from the exposed hook-and-loop fasteners, as well as adherence of the skin layers to each other. We recommend a layer of wax paper be placed between the devices if they are stacked. These devices should be protected from sunlight.
Finally, these devices can only display one grade of pitting edema per unit. To simulate more or less severe grades, additional devices would need to be manufactured with differing volumes of maltose gel in the enclosure.
Conclusion
The lower extremity edema moulage application successfully met the goals of tactile and visual realism and demonstrated excellent durability and usability. Through careful material selection, iterative prototyping, and initial testing, we created a functional and cost-effective training tool that accurately simulates Grade 2+ to 3+ pitting edema. Our device demonstrates appropriate rebound characteristics, passed durability testing, and received overwhelmingly positive feedback from medical students and faculty, highlighting significant improvements over the commercially available foam-based device. The final design integrates multiple strategically integrated components – including a hydrogel core, nanotape sealing, silicone interface, tattoo skin outer layer, and elastic securing straps – to deliver a cohesive, realistic simulation experience. Our solution balances realism and functionality while staying within budget and remaining easy to independently manufacture and maintain.
Supplemental Digital Content Legend
Supplemental Digital Content 1: Online document, “Lower Extremity Edema Moulage Application: Fabrication Protocol” is included with step-by-step instructions and images to assist in replication of this item:
References
Calzon ME, Blebea J, Pittman C. Quantitative measurement of pitting edema with a novel edema ruler. J Vasc Surg Cases Innov Tech. 2023 Nov 21;10(1):101373. https://doi.org/10.1016/j.jvscit.2023.101373
Huang, G.C., Sacks, H., Devita, M., Reynolds, R., Gammon, W., Saleh, M., Gliva-McConvey, G., Owens, T., Anderson, J., Stillsmoking, K., Cantrell, M., & Passiment, M. (2012).
Characteristics of simulation activities at North American medical schools and teaching hospitals: an AAMC-SSH-ASPE-AACN collaboration. Simulation in Healthcare, 7(6), 329-333. http://doi.org/10.1097/SIH.0b013e318262007e
INACSL Standards Committee (2016). INACSL standards of best practice: simulation design. Clinical Simulation in Nursing, 12(S), S5-S12. http://doi.org/10.1016/j.ecns.2016.09.005
Issenberg, S.B., Mcgaghie, W.C., Petrusa E.R., Gordon, D.L., & Scalese, R.J. (2005). Features and uses of high-fidelity medical simulations that lead to effective learning: a BEME systematic review. Medical Teacher, 27(1), 10–28. http://doi.org/10.1080/01421590500046924
Rudolph, J.W., Raemer, D.B., & Simon R. (2014). Establishing a safe container for learning in simulation: the role of the presimulation briefing. Simulation in Healthcare, 9(6), 339-49. http://doi.org/10.1097/SIH.0000000000000047